AscellaHealth HUB Partnership with Abeona Therapeutics® Supports Launch Success of Novel Cell-Based Gene Therapy and Access to Treatment for Rare Disease Patients
BERWYN, Pa., July 29, 2025 (GLOBE NEWSWIRE) -- AscellaHealth, a global partner delivering customizable solutions to support the specialty pharmaceutical industry, highlights the value of its HUB partnership with Abeona Therapeutics Inc. (Nasdaq: ABEO) in the successful pre-and-post launch commercialization of ZEVASKYN™, an FDA-approved cell-based gene therapy. Working as a collaborative partner, AscellaHealth designed and executed patient-centric, end-to-end solutions to address unique clinical, operational and reimbursement needs for a new-to-world autologous cell-based gene therapy. By focusing on the requirements of patients throughout the entire therapeutic journey, AscellaHealth led the collaborative effort to build and launch AbeonaAssist™, a highly customized patient support program that creates a seamless experience for patients, caregivers and healthcare providers.
"Abeona’s groundbreaking cell-based gene therapy demanded a HUB partner who could build tailored infrastructure from the ground up,” said William White, Vice President of Market Access at Abeona Therapeutics. “AscellaHealth’s comprehensive expertise in navigating this complex journey, from patient and provider engagement to optimizing reimbursement for this first-of-its-kind treatment, made them the unequivocal partner of choice. Since launch, their unwavering commitment to a patient-centric model, which perfectly aligns with our program’s vision, has proven invaluable.”
Bill Oldham, Chairman and President, AscellaHealth, says, “We value our strategic partnership with Abeona which is built upon an aligned vision, a foundation of shared goals and a clear understanding of what can be achieved through collaboration. Together, we have created synergies that not only benefit both organizations, but most importantly, patient access to treatment and optimized clinical outcomes. Abeona epitomizes our description of an ideal partner that is committed to open communications, trust and transparency.”
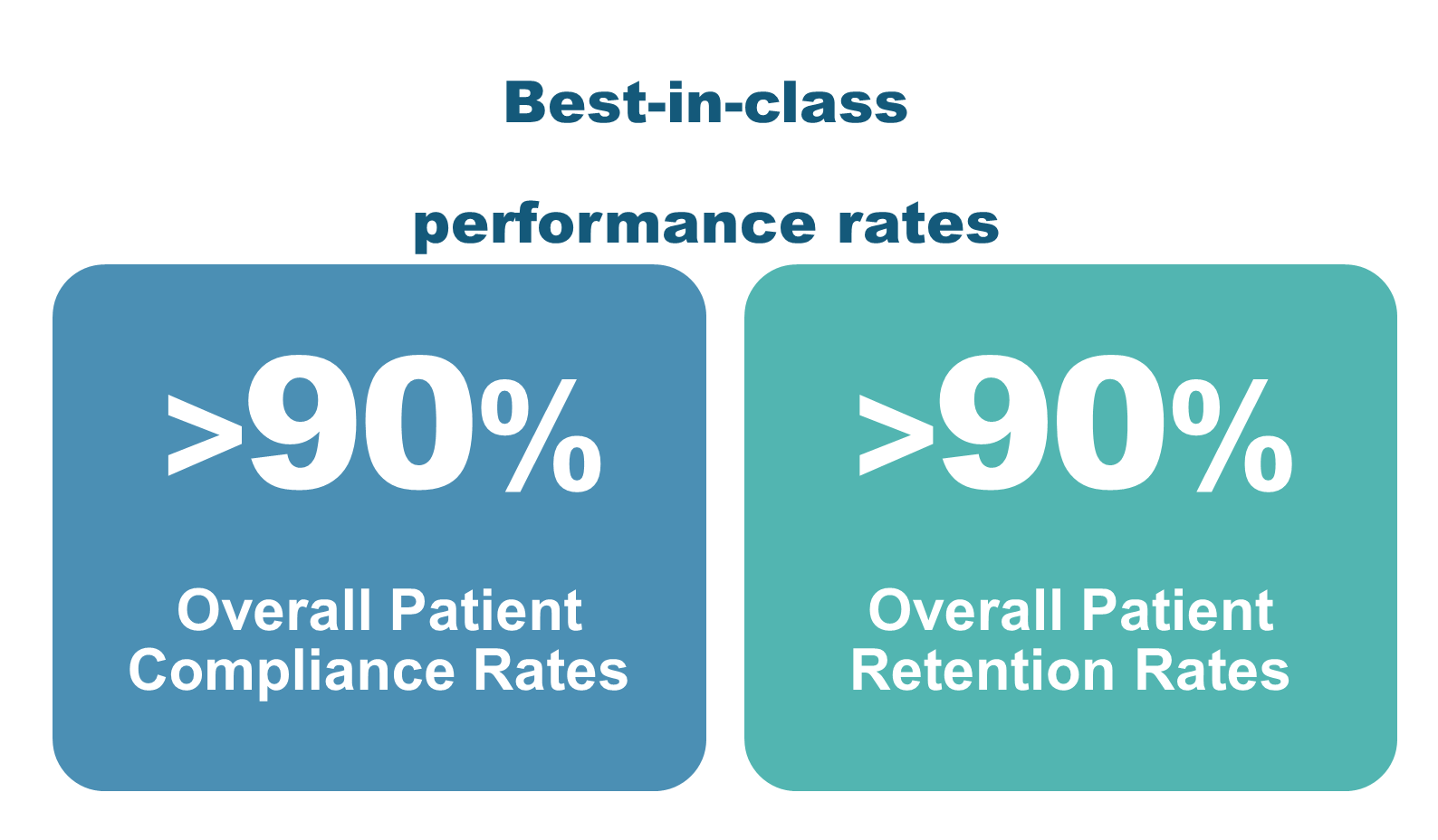
AscellaHealth’s integrated patient services and HUB model are proving instrumental in achieving optimal results for life science manufacturers launching specialty pharmaceuticals, and cell and gene therapies for individuals with complex, chronic conditions and rare disease. The results highlight enhanced compliance, retention and satisfaction rates, underscoring the effectiveness of AscellaHealth’s tailored programs that consistently deliver measurable outcomes:
About ZEVASKYN™ (prademagene zamikeracel) gene-modified cellular sheets
ZEVASKYN is the first and only autologous cell sheet-based gene therapy for the treatment of wounds in adult and pediatric patients with recessive dystrophic epidermolysis bullosa (RDEB). RDEB is a severe skin disease caused by a defect in both copies of the COL7A1 gene resulting in the inability to produce functional type VII collagen. Without functional type VII collagen and anchoring fibrils, the skin is fragile and blisters easily, leading to wounds that continually open and close, or fail to heal altogether. Patients often have large open wounds that can lead to serious life-threatening complications. ZEVASKYN incorporates the functional type VII collagen-producing COL7A1 gene into a patient’s own skin cells, ex vivo, using a replication-incompetent retroviral vector to produce functional type VII collagen in treated wounds. ZEVASKYN has demonstrated clinically meaningful wound healing and pain reduction with a single surgical application. For more information, visit www.ZEVASKYN.com.
Indication
ZEVASKYN™ (prademagene zamikeracel) is an autologous cell sheet-based gene therapy indicated for the treatment of wounds in adult and pediatric patients with recessive dystrophic epidermolysis bullosa (RDEB).
Important Safety Information
- Serious allergic reactions to ZEVASKYN can occur. Patients should get medical help right away if they experience symptoms like itching, swelling, hives, difficulty breathing, runny nose, watery eyes, or nausea. In rare cases, a severe reaction called anaphylaxis may happen.
- There is a potential risk that treatment with ZEVASKYN may contribute to the development of cancer because of how the therapy works. Patients should be monitored for the rest of their lives to check for any signs of cancer.
- ZEVASKYN is made using human and animal materials. Although these materials are tested before use, the risk of passing on infections cannot be eliminated.
- The most common side effects are pain from the procedure and itching.
This is not a complete list of side effects. Patients should call their care team for medical advice about side effects. Side effects may be reported to Abeona at 1-844-888-2236 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See full Prescribing Information.
About AscellaHealth
AscellaHealth is a global partner that delivers proven end-to-end solutions to both life sciences and healthcare companies to enhance the quality of life for patients with complex, chronic conditions. A dedicated team gets critical healthcare products from manufacturers to patients while ensuring an efficient flow of funds between payers and pharma. Visit www.AscellaHealth.com.
About Abeona Therapeutics
Abeona Therapeutics Inc. is a commercial-stage biopharmaceutical company developing cell and gene therapies for serious diseases. Abeona’s ZEVASKYN™ (prademagene zamikeracel) is the first and only autologous cell-based gene therapy for the treatment of wounds in adults and pediatric patients with recessive dystrophic epidermolysis bullosa (RDEB). The Company’s fully integrated cGMP cell and gene therapy manufacturing facility in Cleveland, Ohio serves as the manufacturing site for ZEVASKYN commercial production. The Company’s development portfolio features adeno-associated virus (AAV)-based gene therapies for ophthalmic diseases with high unmet medical need. Abeona’s novel, next-generation AAV capsids are being evaluated to improve tropism profiles for a variety of devastating diseases. For more information, visit www.abeonatherapeutics.com.
ZEVASKYN™, Abeona Assist™, Abeona Therapeutics®, and their related logos are trademarks of Abeona Therapeutics Inc.
Media:
Caroline Chambers
CPR Communications
cchambers@cpronline.com
201.641.1911 x 21
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/15275ef8-6da1-4059-8c83-4c6124d577d1
This press release was published by a CLEAR® Verified individual.

Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.


